740Y-P PI3K Activator | PDGFR-Mediated Phosphoinositide 3-Kinase Stimulation
ABSTRACT
Proper migration and invasion of trophoblast cells into endometrium is vital for successful embryo implantation during early pregnancy.Benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) is an ultimate carcinogenic product of benzo[a]pyrene (BaP), which causes multiple trophoblast-related diseases. However, the mechanism of BPDE-inhibited migration/ invasion of trophoblast cells is still unclear. In this work, we found that BPDE significantly inhibited the filopodia formation and migration/invasion of human trophoblast Swan 71 cells. BPDE up-regulated the level of miR-194-3p, which further inhibited the PI3K/AKT/CDC42/PAK1 signaling pathway and depressed the filophdia formation of Swan71 cells.
Addition of 740 Y-P , the activator of PI3K, could stimulate cell migration/invasion, confirming the involvement of this pathway. Knock-down of miR-194-3p up-regulated this pathway and promoted filopodia formation and migration/invasion. Conversely, overexpression of miR-194-3p down-regulated this pathway and inhibited cell migration/invasion. Therefore, miR-194-3p takes important roles in the BPDE-inhibited filopodia formation and cell migration/invasion, providing valuable information in the BPDE-induced dysfunctions of human extravillous trophoblast cells.
Keywords: BPDE; Human Trophoblast Swan 71 cell; migration and invasion; filopodia formation; PI3K/AKT/CDC42/PAK1 pathway; miR-194-3p
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are produced from incomplete combustion of organic materials and fossil fuel in motor vehicles, residential heating units, power plants, and industries (Bostrom et al., 2002). PAHs are also present in tobacco smoke and are produced from grilling or broiling of food (Stahl et al., 2004). Benzo(a)pyrene (BaP) is a representative of PAH and classified as carcinogen to human by the International Agency. BaP can be absorbed via the oral, inhalation and dermal routes of exposure (Jedrychowski et al., 2017). After absorption by human, BaP is metabolically activated by the aromatic hydrocarbon receptor (AhR)-induced cytochrome P4501A1 (CYP1A1) and epoxide hydrolase, resulting in the formation of carcinogenic benzo(a)pyren-7,8-dihydrodiol-9,10-epoxide (BPDE) (Admiraal et al., 2017). The electrophilic BPDE is capable of covalent binding to cellular macromolecules, such as DNA, RNA, and proteins, which subsequently causes deleterious effects in susceptible tissues (Ling et al., 2004).
Numerous studies have demonstrated that BaP (or BPDE) has serious toxic effects on various systems (Bulay et al., 1971; Rice et al., 1982). In recent years, increasing attention has focused on the effects of BaP on reproductive health. Women exposure to the elevated levels of PAHs, as indicated by the increased PAH-DNA adducts in cord blood, results in the reduced fetal growth (Perera et al., 2005).
In animal experiments, estradiol concentrations are significantly reduced after BaP exposure in female mice, and decidualization and decidual angiogenesis are inhibited by oral BaP ingestion (Admiraal, et al., 2017). These results show that BaP can cross the placenta and be transferred from mother to fetus, resulting in fetal and placental developmental toxicity during early pregnancy. However, the interaction targets and their molecular mechanism are still unclear.
Placentation is crucial to embryo and fetal development. The fundamental steps for successful placentation involve the trophoblast invasion to establish and maintain feto-placental vasculature (Huppertz, 2007; Wulff et al., 2003). Recently, this group has found that BPDE has a significant toxic influence on human extravillous trophoblast cells. BPDE induces human extravillous trophoblast Swan 71 cell dysfunctions due to cell apoptosis through the disorder of mitochondrial fission/fusion (Wang et al., 2018). BPDE also suppresses the migration and invasion of human extravillous trophoblast HTR-8/SVneo cells by down-regulating MMP2 through inhibition of FAK/SRC/PI3K/AKT pathway (Wang, et al., 2017).
Filopodia extend beyond the leading edge of cells during trophoblast invasion. The extension of filopodia is dependent on the elongation of actin filaments toward the plasma membrane (Admiraal, et al., 2017; Arjonen et al., 2011). Filopodia formation is highly regulated by various signaling pathways. Several proteins, such as WASP-family proteins, actin-related proteins 2 and 3 (Arp2/3) (Matsuda et al., 2017), insulin receptor phosphotyrosine 53-kDa substrate (IRSp53) (Lee et al., 2017), and eukaryotic elongation factor 1α (EF1A) (Saisongkorh et al., 2016), can promote actin polymerization and filopodia formation. PI(3,4)P2 and PI(3,4,5)P3, the products of PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), recruit these proteins onto the leading edge of cells to facilitate the actin polymerization (Han et al., 2016). PI3K/AKT pathway is associated with cell migration/invasion (Wang et al., 2017).
AKT, the serine-threonine protein kinase, which can be activated in a PI3K-dependent manner, can also stimulate the filopodia formation (Saisongkorh, et al., 2016). CDC42, a Rho-family GTPase, regulates actin assembly and controls filopodia formation (Huang et al., 2015). CDC42 expression is up-regulated by activating PI3K/AKT pathway, thus promoting the migration of glioblastoma cells (Aad et al., 2015; Maiuthed et al., 2014).
The enrichment of PI(3,4)P2 and PI(3,4,5)P3 on the leading edge is required for CDC42 activation to promote the directional actin polymerization. PAK1, which is a critical factor that links Rho GTPases to cytoskeleton reorganization, serves as targets for small GTP binding protein CDC42. The mRNA level of PAK1 is regulated by PI3K/AKT signaling pathway in lung cancer cells (Wu et al., 2016). PAK1 protein level is elevated in the choriocarcinoma JEG-3 and JAR cells and in trophoblastic tumor; while knockdown of PAK1 in JEG-3 and JAR cells reduces cell proliferation, migration, and invasion abilities (Siu et al., 2010). Based on these results, we speculate that PI3K/AKT/CDC42/PAK1 signaling pathway would facilitate filopodia formation and promote cell migration and invasion.
MicroRNAs predominantly negatively regulate gene expression by binding to the 3’-untranslated region (3’-UTR) of the target genes (Lai, 2002). MicroRNAs participate in the regulation of various cellular processes, such as cell proliferation, cell cycle, and apoptosis (Ambros, 2001; Davalos et al., 2010; Melo et al., 2011). Emerging evidence has demonstrated that miRNAs also take critical roles in the
control of reproductive functions, especially in the processes of oocyte maturation, folliculogenesis, corpus luteum function, implantation, and early embryonic development (Imbar et al., 2014b). Based on our recent work, BPDE inhibits the invasion of human extravillous trophoblast Swan 71 cells (Wang, et al., 2018). Subsequently, all miRNAs in the BPDE-treated Swan 71 cells were analyzed by RNA-sequencing. We found that miR-194-3p was significantly up-regulated after BPDE treatment.
It has been reported that miR-194-3p was down-regulated in spontaneous intestinal perforation tissues compared with surgical control tissues, indicating that the dysregulation of miR-194-3p may involve in SIP (Spontaneous Intestinal Perforation) pathophysiology (Ng et al., 2015b). In this work, we expect to investigate the roles of miR-194-3p in the filopodia formation and migration/invasion of Swan 71 cells after BPDE treatment. Our results show that the miR-194-3p down-regulated PI3K/AKT/CDC42/PAK1 pathway and inhibited filopodia formation, thus suppressing the migration and invasion of BPDE-treated Swan71 cells.
MATERIALS AND METHODS
Materials. Benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) was purchased from MRIGlobal (MRIGlobal, MO, USA). Dimethysulfoxide (DMSO) was from Sigma (St Louis, MO, USA). BPDE was dissolved in DMSO and stored at -80 oC. The final concentration of DMSO in the culture media was lower than 0.1%. All vehicle control and treated cultures contained the same amount of DMSO.
Cell culture. Human trophoblast Swan 71 cells were cultured in DMEM/F12 supplemented with 10% (v/v) FBS, 100 mM Sodium Pyruvate Solution, and MEM Non-Essential Amino Acids Solution at 37 oC in a saturated humidity atmosphere containing 95% air and 5% CO2. All these reagents were from Gibco Life Technologies (GIBCO, Life Technologies, Carlsbad, CA, USA). Swan71 cells in complete medium were seeded in 6-well plates or 96-well plates for 24 h, and then incubated in a new medium containing 1% FBS overnight. Control cells were exposed to vehicle alone (0.01% DMSO in 10% FBS, DMEM-F12 medium). Subsequently, these cells were exposed to BPDE for 24 h. Control cells were exposed to vehicle alone (0.01% DMSO in 10% FBS, DMEM-F12).
Cell viability detection. The viability of Swan 71 cells after exposure to BPDE was determined by MTT assay. MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) was dissolved in phosphate-buffered saline (PBS) and stored at -20 oC. Swan71 cells were seeded in 96-well flat-bottomed plates (Corning, NY, USA) at a density of 4×103/well, incubated overnight, and then exposed to different concentrations (0, 0.25, 0.5, 1.0, 1.5, or 2.0 µM) of BPDE for 24 h. After addition of 20 µl MTT solution (5 mg/ml) to each well, cells were incubated for 4 h. The supernatant was cleared, 150 µl DMSO was added to each well, and the cells were vibrated for 10 min. The optical density was measured at 450 nm using a microplate reader. All experiments were replicated thrice.
High‑throughput RNA sequencing. The HiSeq 2000 sequencing platform (BGI-Shenzhen, Shenzhen, China) was used for high-throughput RNA sequencing. The protocol involved the removal of rRNA, followed by synthesis of double-stranded cDNA and end repair. After linking sequencing adaptors and selecting fragments, the second strand of cDNA was degraded and the remaining strand was enriched by PCR. The quality of the library was confirmed by sequencing. The raw sequencing data was analyzed by bioinformatics. Differentially expressed miRNAs were searched in the NCBI database to determine their genome loci.
Cell transfection. MiR-194-3p inhibitor, its mimics, and negative control were purchased from Life Technologies (Life Technologies, Carlsbad, CA, USA). Swan71 cells were cultured in a 6-well plate for 24 h and the cell culture medium was replaced with OPTI-MEM I (GIBCO, Life Technologies, Carlsbad, CA, USA). The transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The negative control, miR-194-3p inhibitor (siRNA), or its mimics (50 nM) was mixed with lipid carrier (1:1, v/v). Then, the Swan71 cells were incubated in this mixture in OPTI-MEM I medium (GIBCO) for 6 h, or then switched in the growth medium containing 0.5 µM BPDE and incubated for another 24 h. The transfection efficiency was evaluated by determination of miR-194-3p level by real-time PCR.
Determination of migration and invasion by trans-well assays. The effects of BPDE and miR-194-3p on migration and invasion of Swan71 cells were assessed through trans-well assays. Assays were carried out using 24-well trans-well inserts equipped with 8 µm pore size membrane (Corning, Lowell, MA, USA). Swan71 cells were treated with BPDE (0, 0.25, 0.5, 1.0, or 1.5 µM) for 24 h, or transfected with negative control, miR-194-3p inhibitor, or its mimics (+NC, +siRNA, or +mimics), or then treated with 0.5 µM BPDE for 24 h (+NC+BPDE, +siRNA+BPDE, or +mimics+BPDE). The dead cells were removed and only the survived cells were used for the following assays. The survived cells (3×104) were seeded into the upper chamber, and cell migration and invasion were detected. DMEM-F12 media containing 10% FBS was used as chemoattractant in the lower chamber.
After 24 h, the upper chambers were removed, and the cells on the upper side of the membrane were completely removed through gentle swabbing. The remaining cells that migrated or had invaded into the membrane were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with 0.5% crystal violet. The migrated cells in each well were counted under a phase contrast microscope. For invasion assay, cells were placed into the upper chamber, and each membrane was coated with 70 µl matrigel (BD Biosciences, Franklin Lakes, NJ, USA). All experiments were replicated thrice.
Wound-healing assays. The effect of miR-194-3p on migration of Swan71 cells was assessed by cell wound-healing assay. Swan71 cells (5×104) were transfected with negative control or miR-194-3p inhibitor (+NC or +siRNA), or then treated with 0.5 µM BPDE for 24 h (+NC+BPDE or +siRNA+BPDE). The dead cells and remaining BPDE were removed. The survived cells were incubated in 6-well plates until 80% confluence. A 20 µl tip was used to generate scratch-wound. Swan71 cells were incubated in serum-free DMEM-F12 media (GIBCO) for 12 or 24 h and were photographed. The wound areas were count with Image J software (National Institutes of Health, Bethesda, MD, USA). All experiments were replicated thrice.
Transmission electron microscopy. Swan 71 cells treated with BPDE (0, 0.5, or 1.0 µM) for 24 h were fixed with 4% glutaraldehyde and post-fixed in a 1% osmium tetroxide (OsO4) solution for 1 h at 4 °C. The samples were washed, dehydrated in a graded alcohol series, and embedded in Epon-Araldite resin. Ultra-thin sections (50 nM) were obtained using an ultramicrotome (Leica, Wetzlar Germany). The sections were stained with uranyl acetate and lead citrate. The cellular filopodia were observed on a Hitachi H-7500 transmission electron microscope (Hitachi, Ltd. Tokyo, Japan).
Quantitative real-time polymerase chain reaction (Q-RT-PCR). Swan71 cells (5×104) were treated with BPDE (0, 0.25, 0.5, 1.0, or 1.5 µM) for 24 h, or transfected with negative control or miR-194-3p inhibitor (+NC or +siRNA), or then treated with 0.5 µM BPDE for 24 h (+NC+BPDE or +siRNA+BPDE). Total RNA in cells was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA was generated from 1 µg total RNA using the PrimeScript RT reagent Kit (Promega, WI, USA) for Q-RT-PCR analysis of the mRNA levels of PI3K, CDC42, AKT, and PAK1.
For miR-194-3p, total RNA (2 µg) was transcribed into cDNA using a microRNA First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. All the primers used in Q-RT-PCR were listed in Table 1. Quantitative detection was carried out using an IQ5 real-time detection system (Bio-Rad Laboratories, Hercules, CA) and SYBRVR Premix Ex TaqTM II (Takara, Dalian, China). GAPDH was used as the normalization internal standard. The results were analyzed with the 2-∆∆CT method. All experiments were replicated thrice.
Western blotting analysis. Swan71 cells (5×104) were treated with BPDE (0, 0.25, 0.5, 1.0, or 1.5) for 24 h, or transfected with negative control, miR-194-3p inhibitor or its mimics (+NC, +siRNA, or +mimics), or then treated with 0.5 µM BPDE for 24 h (+NC+BPDE, +siRNA+BPDE, or +mimics+BPDE).
Total proteins were extracted and their concentrations were determined by BCA method. Equal amounts (20 µg) of cellular proteins were separated on 8%-12% SDS-PAGE gel and transferred onto equilibrated polyvinylidene difluoride membrane (Amersham Biosciences, Buckinghamshire, UK). After blocking with 5% skimmed milk in TBST (20 mM Tris-HCl, 1.5 M NaCl, and 0.1% Tween-20) for 1 h at room temperature, the membrane was incubated with primary antibody in blocking solution at 4 oC overnight. Primary antibody against PI3K, AKT, CDC42, or PAK1 was purchased from Abcam (ab32089, ab81283, ab187643, or ab40795, respectively, 1:800) (Abcam, Cambridge, MA, USA). The membrane was washed thrice in TBST for 15 min and incubated with secondary antibody in blocking solution for 1 h at room temperature.
Proteins were detected by enhanced chemiluminescence (Amersham Corporation, Arlington Heights, IL, USA). GAPDH was used as internal standards. All experiments were replicated thrice.
740 Y-P stimulation. 740 Y-P (PDGFR 740 Y-P) with molecular formula of C141H222N43O39PS3 (Fig. 5A) was found as an activator of PI3K in human melanoma MNT-1 cells (Bin et al., 2014). To confirm the roles of PI3K/AKT/CDC42/PAK1 pathway in cell migration/invasion, Swan71 cells or cells exposed to 0.5 µM BPDE for 6 h were treated with 10 µM 740 Y-P for 24 h. Then, cell migration/invasion, and the protein expression levels in this PI3K/AKT/CDC42/PAK1 pathway were determined as described above. The Swan71 cells or the BPDE-treated cells in the absence of 740 Y-P were also determined as control samples. All experiments were replicated thrice.
Statistical analysis. Data were analyzed using SPSS 18.0 software (SPSS Inc, Chicago, IL, USA) and expressed as means ± standard deviation. Results of cell migration and invasion, trans-well, wound-healing, and Q-RT-PCR were evaluated using the one-way analysis of variance (ANOVA). Differences were considered significantly when p*< 0.05 or p**< 0.01.
RESULTS
BPDE Inhibited Swan71 Cell viability
To determine whether BPDE affects the Swan 71 cell viability, the viability was determined after BPDE treatment. Based on the occupational exposure, diffusion, and accumulation of BaP and its metabolite in body, as well as our previous studies (Wang, et al., 2017; Wang, et al., 2018), Swan 71 cells were exposed to 0, 0.25, 0.5, 1.0, 1.5, or 2.0 µM BPDE for 24 h. The viability of Swan71 cells gradually decreased with increasing BPDE concentration in a dose-dependent manner. BPDE above 0.50 µM significantly inhibited cell growth and viability. The cell viability decreased by nearly 50% relative to the control cells in the presence of 1.5 µM BPDE (Fig. 1). Thus, we selected the condition for treatment of Swan 71 cells with 0.5 µM BPDE for 24 h in dose-dependent assays and with 0.5 µM BPDE for transfection assays and 740 Y-P stimulation assays.
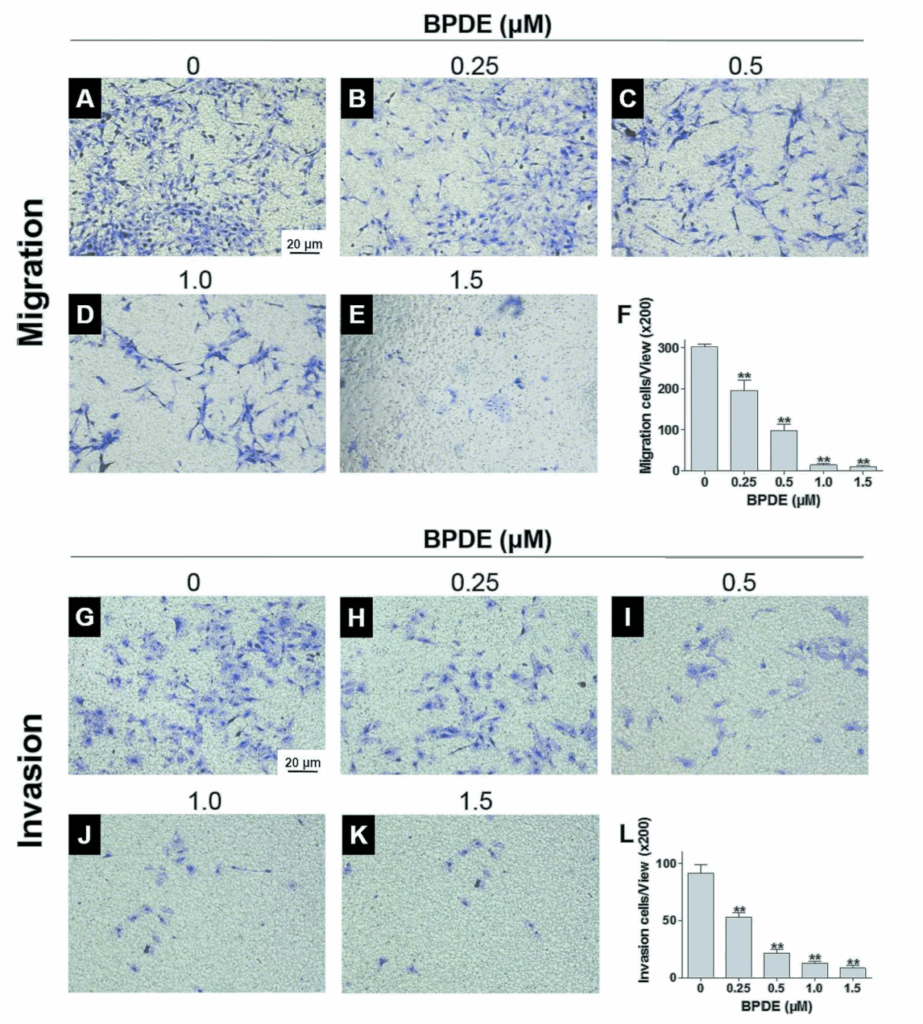
BPDE inhibits the migration and invasion of Swan71 cells.
Swan 71 cells have migration and invasion functions for successful placentation. To determine the effects of BPDE on cell migration, the dead cells were removed and only the survived cells were used for the following experiments. The migration ability of Swan71 cells that had been exposed to 0, 0.25, 0.5, 1.0, or 1.5 µM BPDE was determined through trans-well assays (Fig. 2A-E). Untreated Swan71 cells easily migrated through the membrane, as observed that the cells were clearly strained by crystal violet.
The number of the migrated cells decreased with increasing BPDE concentrations in a dose-dependent manner. The cell migration ability was significantly inhibited with 1.0 and 1.5 µM BPDE (Fig. 2F). The effect of BPDE on cell invasion was also investigated using trans-well assays (Fig. 2 G-K). The untreated Swan71 cells readily invaded into the membrane. However, the invasion ability significantly decreased with increasing BPDE concentration in a dose-dependent manner (Fig. 2L, p** < 0.01). Overall, BPDE inhibited the migration and invasion of Swan 71 cells.
BPDE inhibits the formation of filopodia.
Filopodia take important roles in cell migration and invasion. Swan71 cells were treated with different concentrations of BPDE (0, 0.5, or 1.0 µM) for 24 h. The filopodia of the BPDE-treated Swan 71 cells were directly visualized using TEM (Fig. 3 A-F). The number and length of cell filopodia decreased at 0.5 µM BPDE and further decreased at 1.0 µM BPDE compared with the control cells. All the number and length of filopodia were quantified and plotted against the BPDE concentration (Fig. 3G, H, p**< 0.01), showing that BPDE significantly inhibited the filopodia formation in a dose-dependent manner.
BPDE inhibited the PI3K/AKT/CDC42/PAK1 signaling pathway.
BPDE inhibited the formation of cell filopodia (Fig. 3).
PI3K/AKT/CDC42/PAK1 pathway involves in the filopodia formation (Fig. 4A). To determine whether BPDE inhibits the filopodia formation through this pathway, the protein and mRNA levels of PI3K, AKT, CDC42, and PAK1 were analyzed by western-blot (Fig. 4B) and RT-PCR (Fig. 4C-F) assays, respectively. Compared with the untreated cells, proteins levels of PAK1, PI3K, AKT, and CDC42 were significantly decreased with increasing BPDE concentrations in a dose-dependent manner (p < 0.05, p< 0.01). Their mRNAs levels were also significantly decreased in a dose-dependent manner (p < 0.05, p< 0.01). These data show that BPDE inhibited filopodia formation through the PI3K/AKT/CDC42/PAK1 pathway.
740 Y-P promoted the migration and invasion of Swan71 cells by activation of the PI3K/AKT/CDC42/PAK1 signaling pathway
To further confirm the involvement of this signaling pathway in migration/invasion of the BPDE-treated cells, Swan71 cells were treated with 10 µM 740 Y-P, an activator of PI3K (Fig. 5A). The protein level of PI3K was up-regulated after 740 Y-P treatment in Swan71 cells or in the BPDE-treated cells (Fig. 5B). The protein levels of its downstream AKT, CDC42, and PAK1 were also up-regulated (Fig. 5B). Cell migration and invasion were also promoted by 740 Y-P stimulation in Swan71 cells or in the BPDE-treated cells (Fig. 5C-F, p < 0.05). All these data showed that BPDE inhibited the migration/invasion of Swan71 cells by inhibition of the PI3K/AKT/CDC42/PAK1 signaling pathway.
MiR-194-3p was up-regulated in BPDE-treated Swan71 cells.
All microRNAs in the BPDE-treated Swan71 cells were analyzed by high throughput RNA sequencing. The expression levels of 24 miRNAs were significantly up-regulated and 21 were down-regulated after BPDE treatment (fold change > 2 and p < 10-3). Among them, miR-194-3p (Fig. 6A) was one of the significantly up-regulated miRNAs (Fig. 6B). Q-RT-PCR results further confirmed that miR-194-3p was up-regulated in the BPDE-treated Swan 71 cells (Fig. 6C).
To investigate the roles of miR-194-3p in migration/invasion, miR-194-3p was knocked-down or overexpressed in Swan 71 cells (Fig. 6A,D). In details, Swan 71 cells were transfected with negative control, its inhibitor, or its mimics (+NC, +siRNA, or +mimics), or then treated with 0.5 µM BPDE (+NC+BPDE, +siRNA+BPDE, or +mimics+BPDE) (Fig. 6D). The miR-194-3p level was detected by q-RT-PCR to evaluate the transfection efficiency. The miR-194-3p levels were similar for the untreated (0) and negative control (+NC) cells, showing that the transfection of negative control did not affect the miR-194-3p level. Transfection of cells with its siRNA significantly knocked down the miR-194-3p level and transfection with its mimics significantly up-regulated its level in Swan 71 cells or in the BPDE-treated cells (Fig. 6D).
Down-regulation of miR-194-3p promoted the migration and invasion of Swan71 cells
Since miR-194-3p level was up-regulated in the BPDE-treated Swan71 cells, it was expected that miR-194-3p would inhibit the migration and invasion of Swan71 cells. To explore this possibility, Swan 71 cells were transfected with negative control or miR-194-3p inhibitor (+NC or +siRNA), or then treated with BPDE (+NC+BPDE or +siRNA+BPDE), and their migration and invasion were examined (Fig. 7A,B).
The migration and invasion were similar for the untreated (0) and negative control
(+NC) cells, showing that negative control had no obvious effect on cell migration and invasion. The migration and invasion were obviously promoted by knocking down miR-194-3p level in Swan 71 cells or in the BPDE-treated cells. The number of migrated and invaded cells was counted and plotted (Fig. 7C,D), confirming that miR-194-3p did significantly inhibit cell migration and invasion.
Wound-healing assays were also performed using Swan 71 cells that were transfected with negative control or miR-194-3p inhibitor (+NC or +siRNA), or then treated with BPDE (+NC+BPDE or +siRNA+BPDE) (Fig. 8). The wound areas were calculated and normalized against that of the negative control cells (Fig. 8B). Knock-down of miR-194-3p significantly promoted the migration of Swan 71 cells or the BPDE-treated cells.
Down-regulation of miR-194-3p promoted the PI3K/AKT/CDC42/PAK1 signaling pathway
As we have confirmed, BPDE down-regulated PI3K/AKT/CDC42/PAK1 pathway and inhibited the filopodia formation and cell migration/invasion. Meanwhile, miR-194-3p was up-regulated by BPDE and inhibited cell migration/invasion. Thus, we further investigate whether miR-194-3p would down-regulate the PI3K/ AKT/ CDC42/PAK1 signaling pathway. To verify this hypothesis, the protein and mRNA levels of PI3K, AKT, CDC42, and PAK1 were detected after down-regulation of miR-194-3p by transfection of Swan 71 cells with its inhibitor (Fig. 9). Similar to the treatments of cells as described above, knock-down of miR-194-3p up-regulated these protein expression levels in Swan 71 cells or in the BPDE-treated cells (Fig. 9A). The similar results were also observed at their mRNA levels (Fig. 9B-E). All these results confirmed that miR-194-3p significantly down-regulated the PI3K/AKT/CDC42/PAK1 signaling pathway, thus inhibiting the filopodia formation and cell migration/invasion.
Overexpression of miR-194-3p inhibited cell migration/invasion and PI3K/AKT/CDC42/PAK1 signaling pathway
MiR-194-3p was also overexpressed in Swan 71 cells by transfection of cells with its mimics to further validate the roles of miR-194-3p in migration/invasion. Swan 71 cells were transfected with negative control or miR-194-3p mimics (+NC or +mimics), or then treated with BPDE (+NC+BPDE or +mimics+BPDE). The migration and invasion significantly decreased after overexpression of miR-194-3p in Swan 71 cells or in the BPDE-treated cells (Fig. 10A-D), further confirming that miR-194-3p did inhibit the migration and invasion. Overexpression of miR-194-3p also down-regulated the protein expression levels of PI3K/AKT/CDC42/PAK1 signaling pathway in Swan71 cells and in the BPDE-treated cells (Fig. 10E). All these results confirmed that miR-194-3p inhibited the filopodia formation and cell migration/invasion via the down-regulation of the PI3K/AKT/CDC42/PAK1 signaling pathway in the BPDE-treated Swan 71 cells.
DISCUSSION
BPDE is an ultimate carcinogenic product of BaP. BaP concentration in ambient air is in the range from 20 to 100 mg/m3 for the occupational and domestic activities such as cooking with oil or wood combustion (Wormley et al., 2004). The total daily intake of BaP is estimated to be 125 ng/person/day based on daily food consumption and the contaminant BaP level (Lee et al., 2007).
The lipophilicity and long half-life of BaP further increase its accumulation up to a high level in body. It was also found that the levels of BaP in the follicular fluid of women who did not conceive (1.79 ± 0.03 ng/ml) were significantly higher than those that achieved a pregnancy (0.08 ± 0.03 ng/ml) (Neal et al., 2008; Ptashekas et al., 1996). For women who smoke 12-24 cigarettes/day, BaP is 4-10 ng/ml in follicular fluid or twice higher in serum relative to non-smokers. The total concentration of BaP and its metabolites is in the range of 40-100 ng/ml (Xie et al., 2010). In this study, considering occupational exposure, diffusion and accumulation of BaP and its metabolites, we selected 0.25-1.5 µM (75-450 ng/ml) BPDE to treat human trophoblast Swan 71 cells and found that the cell migration/invasion were significantly inhibited in this concentration range.
Placentation is critical in the embryo and fetal development. During early pregnancy, proper migration and invasion of trophoblast cells into endometrium is vital for embryo implantation (Wang, et al., 2017). Numerous studies have shown that BPDE causes trophoblast-related diseases, such as preeclampsia, growth restriction or miscarriages. In our recent work, BPDE decreases the protein levels of mitochondrial fusion genes and increases those of fission genes, resulting in the release of Cyt c, activation of Caspase 3, and Swan 71 cell apoptosis (Wang, et al., 2018).
BPDE also significantly inhibits cell migration/invasion of human trophoblast HTR-8/SVneo cells by down-regulating the FAK/SRC/PI3K/AKT/eNOS/MMP2 pathway (Wang, et al., 2017) . Filopodia formation is a crucial process in cell migration and invasion. In oral squamous cell carcinoma SAS and HSC3 cells, the filopodia formation is considered as the prerequisite for cell migration (Yasui et al., 2017). A promoter (PLAC8) for cell migration/invasion could significantly promote the filopodia formation in human extravillous trophoblast HTR-8/SVneo cells (Cheng et al., 2005). In this study, we found BPDE significantly inhibit the filopodia formation and cell migration/invasion in a dose-dependent manner (Figs. 2 and 3).
PI3K/AKT pathway is important for filopodia formation and cell migration/ invasion. Increased PIP3 level could activate AKT via photosensitive-dependent kinase-1. The downstream genes of AKT, such as eNOS, MMP2, MMP9, and F-actin, widely participate in migration and invasion (Chan et al., 1999; Fukumura et al., 2001; Ohshiro et al., 2006; Zegers et al., 2014). PI3K and CDC42 are important in the filopodia formation. CDC42 regulates the assembly and organization of the actin cytoskeleton by binding it to PI3K products (PI(3,4)P2 and PI(3,4,5)P3 (Shekarabi et al., 2002; Vadlamudi et al., 1999).
PAK1 was initially considered as effect molecule of Rho GTPases, Rac, and CDC42 (Manser et al., 1994), and is now increasingly recognized as important mediator for a wide variety of cellular functions, including cell morphogenesis, motility, mitosis, apoptosis, and angiogenesis (Bokoch, 2003; Kumar et al., 2006). PAK1 could be phosphorylated and activated by AKT and CDC42 (Bi et al., 2003). Cell migration and invasion are reduced after knock-down of PAK1 in choriocarcinoma cell-lines JEG-3 and JAR cells (Siu, et al., 2010). In this work, we found BPDE significantly reduces the mRNA and protein levels of PI3K, AKT, CDC42, and PAK1 in a dose-dependent manner, thus impairing the filopodia formation and migration/invasion of Swan 71 cells.
To confirm the involvement of this PI3K/AKT/CDC42/PAK1 pathway in cell migration/invasion, we treated Swan 71 cells with 740 Y-P, the activator of PI3K found in human melanoma MNT-1 cells (Bin, et al., 2014). The protein expression levels of PI3K and its downstream AKT, CDC42, and PAK1 were all up-regulated, accompanied with the promoted the migration/invasion of Swan 71 cells or the BPDE-treated Swan 71 cells, further confirming that BPDE inhibits cell migration/invasion through down-regulating this pathway.
Recently, miRNAs have been shown to play important roles in the control of reproductive functions, especially in the processes of oocyte maturation, implantation, and early embryonic development (Albrecht et al., 2013; Watanabe et al., 2014). The aberrant expression of some specific miRNAs is linked to some certain female reproductive disorders (Galliano et al., 2014; Imbar et al., 2014a). MiR-194-3b is down-regulated in spontaneous intestinal perforation tissues, and its potential target genes (MMP9, TIMP1, and FOSL1), which are involved in migration and invasion, are all up-regulated (Ng, et al., 2015a).
MiR-194-3p is also up-expressed in chronic hepatitis B, but whether miR-194-3b regulates potential target genes or affects cell biological functions is unclear (Ninomiya, et al., 2016). In this work, we found that miR-194-3p, which is up-regulated in the BPDE-treated Swan 71 cells, down-regulates this PI3K/AKT/CDC42/PAK1 pathway and inhibits the filopodia formation and migration/invasion of Swan71 cells. Knock-down of miR-194-3p by transfection with its inhibitor up-regulates this pathway and promotes filopodia formation. Conversely, overexpression of miR-194-3p by transfection with its mimics further down-regulates this pathway and inhibits cell migration/invasion.
After sequencing alignment using miRanda, Targetscan, PITA, and RNAhybrid software, the sequence of miRNA-194-3p cannot be complementary with any region of these mRNA sequences of PI3K, AKT, CDC42, or PAK1, indicating that there should be no direct interaction between miRNA-194-3p and these mRNAs. Thus, we predict that miRNA-194-3p should be indirectly inhibit this pathway by interactions with some others factors.
A model was proposed to describe the roles of miR-194-3p in migration/invasion (Fig. 11). BPDE up-regulates miR-194-3p, which down-regulates PI3K/AKT/ CDC42/PAK1 signaling pathway, and inhibits filopodia formation and weakens cell migration/invasion. 740 Y-P, the activator of PI3K, could promote this signaling pathway and promote cell migration/invasion. Knock-down of miR-194-3p could up-regulate this pathway and promote filopodia formation. Conversely, overexpression of miR-194-3p down-regulates this pathway and inhibits cell migration/invasion.
In summary, BPDE up-regulates the miR-194-3p level, which further inhibits the PI3K/AKT/CDC42/PAK1 signaling pathway and depresses the filophdia formation and migration/invasion of Swan71 cells. These results suggest that miR-194-3p takes important roles in the biological effects of BPDE on human Swan71 cells, providing valuable information in the BPDE-induced dysfunctions of human extravillous trophoblast cells.
Abbreviations: ANOVA, analysis of variance; BaP, benzo[a]pyrene; BPDE, benzo[a]pyren-7,8-dihydrodiol-9,10-epoxide; DMSO, dimethylsulfoxide; EVT, extravillous trophoblast; EGF, epidermal growth factor; eNOS, endothelial nitric oxide synthase; FBS, fetal bovine serum; MMP2, matrix metallopeptidase 2; MMP9, matrix metallopeptidase 9; PAH, polycyclic aromatic hydrocarbon; PBS, phosphate-buffered saline; PI3K, phosphoinositide 3-kinase; PAK1, p21 (RAC1) activated kinase 1; CDC42, cell division cycle 42; PI(3,4,5) P3, PI3,4,5-triphosphate; PI(3,4)P2, PI3,4-bisphosphate.
FIGURE LEGENDS
Fig. 1. BPDE reduced Swan 71 cell viability. Swan 71 cells, a well-characterized human trophoblast cell line, were exposed to varying concentrations of BPDE (0 to 2 µM) for 24 h.pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE)]The cell viability was measured using the MTT assay, a colorimetric method used to assess metabolic activity as an indicator of cell’s health. The values were presented as means ± SD of three independent experiments. A value of p < 0.01 indicates a significant difference between the treated cells and the control cells, a threshold commonly used in biomedical research to ensure statistical significance (National Institutes of Health).