Abstract
Recent work has indicated that smooth mus- cle force production may be influenced by pathways not dependent upon the Ca2+-calmodulin phosphorylation of light chains. Few studies, however, have examined the importance of these pathways in intact muscles that contract phasically rather than tonically. Therefore, to determine whether the Ca2+-independent Rho-A and as- sociated kinase (ROK) pathway can affect contractions of the intact human myometrium, we used Y-27632 to inhibit ROK. Three types of contractile activity were examined: spontaneous and those elicited by oxytocin and by depolarisation by high K+. Y-27632 decreased force significantly under all three conditions, without changing intracellular [Ca2+]. However, the effects on force were only large when the uterus was producing force tonically rather than phasically. This suggests that the Rho-A-ROK pathway may not be a potent modula- tor of force in the human myometrium under physiolog- ical conditions.
Keywords
Uterus · Contraction · Signalling · Phosphatase
Introduction
The activity of the smooth muscle of the uterus is modu- lated so that it is relatively quiescent before term and then contracts powerfully at term. There is much interest in gaining a better understanding of how this modulation is achieved, so that appropriate activity, such as pre-term labour, can be controlled. The main force-producing pathway in the uterus is the Ca2+-calmodulin-myosin light chain (MLC) kinase (MLCK) pathway [3]. Howev- er contraction can be regulated by the phosphorylation state of MLC20, which depends on the balance between MLCK and MLC-phosphatase (MLCP) activation [6]. Agonists, for example, activate the Ca2+-calmodulin- MLCK pathway but may also stimulate force production by inhibiting MLCP via Rho-associated kinase (ROK) phosphorylation [7]. It is unclear how important this pathway is for contractions of the intact myometrium, although RhoA has been reported in the myometrium and to translocate to the cell membrane from the periph- ery upon muscarinic stimulation [2], and ROK expres- sion increases with pregnancy [5]. We therefore investi- gated the effect of inhibiting the Rho-A pathway using Y-27632, a selective inhibitor of ROK [1], on human myometrial contractions, arising either spontaneously or elicited by membrane depolarisation by high [K+] or with oxytocin stimulation. In addition, we measured in- tracellular [Ca2+] ([Ca2+]i) to determine if any functional effects of Y-27632 were occurring independently of changes in [Ca2+]i.
Materials and methods
Human, non-labouring, myometrial tissues were obtained from women undergoing elective Caesarean section at term (37– 41 weeks). Exclusion criteria were serious medical complications or use of medication likely to affect myometrial activity. Written informed consent was obtained and Ethical approval granted. Tis- sue was placed in physiological saline (2 mM Ca2+ pH 7.4) and then strips (1×1×5 mm) of longitudinal myometrium dissected and incubated overnight in Krebs’ solution containing 15 µM Indo-1 for Ca2+ measurements, as described in detail elsewhere [4]. The strips were then mounted in a small chamber on an in- verted microscope, with one end attached to a Grass FT03 force transducer. Experiments were performed at 35 °C and tissues su- perfused with solution throughout. Paired data were analysed with Student’s t-test; differences between means were assumed to be significant at P<0.05. Data are given as means±SEM for n samples. Y-27632 was a kind gift from Yoshitomi Pharmaceutical Industries.
Results
Effects of Y-27632 on spontaneous contractions and [Ca2+]i
Adding Y-27632 (10 µM) to spontaneously active prepa- rations of human myometrium for up to 20 min produced a small (16±2%) but significant decrease in the ampli- tude of contractions compared with control (100%), as shown in Fig. 1, but no significant change in the Ca2+ transient (n=4). The frequency of contractions also in- creased significantly to 162±6%. The rate of relaxation of the contractions increased significantly to 23±2% (n=6), and thus the contractions were significantly shorter.
The effects of Y-27632 on contractions and [Ca2+]i induced by oxytocin
To investigate whether activation of MLCP could influ- ence agonist-induced contraction, the effects of Y-27632 on the response to oxytocin (10 and 100 nM) were stud- ied. As can be seen in Fig. 2, Y-27632 did not abolish the rise in [Ca2+]i or force produced by 10 nM oxytocin (n=4); it only produced a small (12±2%) decrement of force. With 100 nM oxytocin, Y-27632 significantly re- duced the tonic component of force (54±2%, n=3), with- out a decrease in [Ca2+]i (not shown). Adding oxytocin (100 nM) to the uterus in the presence of KCl (Fig. 2B) potentiated force with a small transient rise of [Ca2+]i, indicating release of Ca2+ from internal stores. To estab- lish whether this potentiation of force by oxytocin was due to the modulation of MLCP activity via inhibition of ROK, we applied Y-27632. After 1–2 min, force began to fall markedly. The superimposed traces show that Y-27632 produced a marked decrease of force (85±5%, n=3, compared with the peak amplitude in control traces) to near basal levels without a change in [Ca2+]i.
The effect of Y-27632 on contractions and [Ca2+]i induced by KCl The effect of Y-27632 on KCl-induced contractions was also examined. Figure 3 shows a control response to KCl (40 mM). Force and [Ca2+]i rose and were maintained until return to control solution. After recovery from KCl, the effects of Y-27632 were studied (Fig. 3, superim- posed trace). After 1–2 min exposure to Y-27632 (in the presence of KCl) there was a significant decrease in force without a change in [Ca2+]i. The decrease in the mean maximum force was 53±2% (n=8), compared with control.
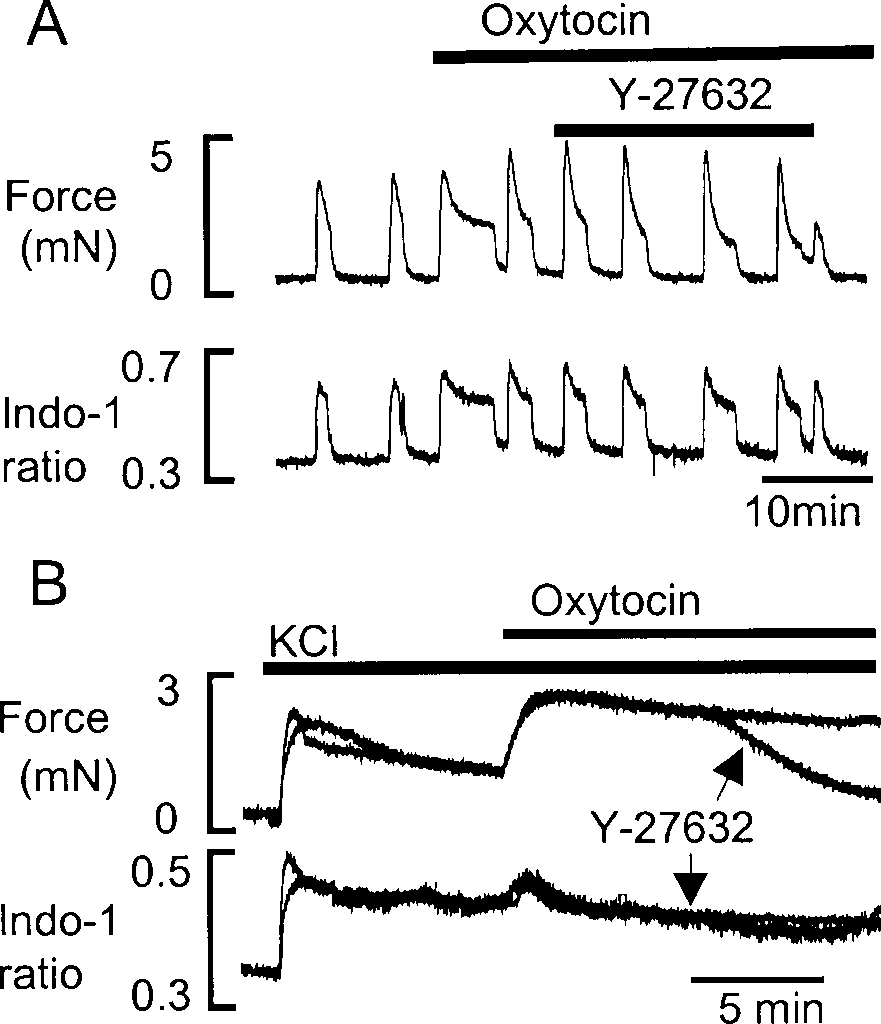
Fig. 1 The effects of Y-27632 on force and Ca2+ signal, in re- sponse to 10 nM oxytocin. B As in A, but with 100 nM oxytocin and the preparation depolarised by exposure to 40 mM KCl. The control trace and that with Y-27632, added after 5 min in oxytocin, were obtained in the same tissue and are superimposed.
Discussion
The amplitude and duration of the normal phasic con- tractions of pregnant non-labouring uterus decreased when ROK modulation of MLCP was inhibited by Y-27632. The degree of modulation was, however, only small, indicating that phasic activity is not greatly en- hanced by this mechanism. These data are consistent with our previous findings that calcium entry and stimu- lation of MLCK is the predominant mechanism modulat- ing spontaneous contractions [3]. The spontaneous con- tractions also shortened due to an increased rate of relax- ation. This suggests that the MLCP was more active, consistent with ROK inhibition by Y-27632.
The augmentation of force and [Ca2+]i in response to physiological concentrations of oxytocin (10 nM) were little affected by Y-27632, consistent again with Ca2+ en- try playing a major role in this kind of contraction. How- ever, when oxytocin was added under depolarised condi- tions or at supra-physiological concentrations (100 nM), and force production occurred with little rise of [Ca2+]i, indicating that a change in the sensitivity to Ca2+ is the major mechanism, then Y-27632 had profound effects. These conditions perhaps also occur in permeabilised preparations in which [Ca2+] is maintained at elevated levels: under such circumstances Y-27632 has large effects on force in rat myometrium [2]. Interestingly, Y-27632 also decreased force in preparations depolarised by high K+, indicating that MLCP modulation may play a role in this type of maintained contraction [8]. Thus the effect of MLCP modulation in vivo and its usefulness as a therapeutic tool will depend upon the relative importance of Ca2+-sensitising pathways, compared with Ca2+ entry mechanisms.
