A B S T R A C T
Belumosudil (BLM) is a ROCK inhibitor that has been firstly developed by Surface Logix, later acquired by Kadmon Pharmaceuticals for the treatment of chronic graft-versus-host disease (cGVHD), Psoriasis Vulgaris (PV), idiopathic pulmonary fibrosis (IPF), hepatic impairment (HI), diffuse cutaneous systemic sclerosis (dcSSc). BLM received a breakthrough therapy designation and priority review from the FDA, which reviewed the NDA under the real-time oncology review (RTOR) pilot programme and approved it six weeks ahead of the PDUFA deadline of August 30, 2021. On July 16th, 2021, The USFDA authorized BLM under the brand name REZUROCKTM for the treatment of cGVHD in adults and pediatric patients aged ≥ 12 years after the fail- ure of at least two prior lines of systemic therapy. It has been granted orphan drug status by the FDA on August 9, 2020, for the treatment of systemic sclerosis. The European Union (EU) granted Quality Regulatory Clinical Ireland Limited, Ireland, orphan drug status for BLM (KD025) for the treatment of cGVHD on October 17, 2019. BLM is under regulatory assessment by Therapeutic Good Administration (TGA) Australia, Health Canada, MHRA (UK), and The Swiss Agency for Therapeutic Products (Swissmedic), Switzerland for cGVHD. A clinical trial is ongoing in the United States for cutaneous systemic sclerosis. This review article summarizes the milestones in the development of BLM chemistry, Chemical synthesis and development, mechanism of action, pharmacokinetics (PK), pharmacodynamics (PD), adverse effects, regulatory status, and ongoing clini- cal trials (CT) of BLM.
Introduction
Belumosudil KD025 ROCK inhibitor is a kinase inhibitor being developed by Kadmon Pharmaceuticals for the treatment of cGVHD in adults and pediatric patients aged ≥ 12 years after the failure of at least two prior lines of systemic therapy . BLM act by inhibiting RHO-associ- ated coiled-coil kinase 2 (ROCK-2). BLM bind and inhibit the serine/threonine kinase activity of ROCK-2. This inhibits ROCK-2 mediated signaling pathways, which plays an important role in pro- and anti-inflammatory immune cell responses. BLM was approved by the FDA on 16th July 2021. cGVHD is a main cause of potentially life-threatening survivors of allogeneic hematopoietic stem cell transplantation. Increasing numbers of around elderly patients and peripheral blood stem cell transplant (PBSCT) resulted in more cGVHD. Understanding the pathogenesis of cGVHD and defining precise diagnostic and classification criteria for clinical pre- sentation have recently progressed. Anti-Thymocyte Globulin (ATG) or post-Tx cyclo reduces the incidence of cGVHD.
Agents that are effective in the treatment of autoimmune illnesses have been used as therapy for established cGVHD, with response rates ranging from 20% to 80%. Maximum reactions are limited to the skin, soft tissue, oral mucosa, and infrequently the liver. Bronchiolitis obliterans is a rare condition that responds poorly to treatment and has a poor prognosis. Thalidomide, photopheresis therapy, antitumor necrosis factor, and B cell suppression using anti-CD20 monoclonal antibodies are among the newer potential therapeutic options under consideration . Clinically, cGVHD is a pleiotropic multiorgan dis- ease that is characterized by tissue inflammation and fibrosis, which frequently lead to irreversible organ damage . cGVHD was first described as a condition that manifested more than 100 days after transplantation, 3 types of onsets are known as quiescent (after reso- lution of cGVHD), progressive (direct progression from acute into chronic) and de novo without prior cGVHD . In the United States, an estimated 14,000 individuals are living with cGVHD, with 5,000 new cases identified every year.
The multi-organ complexity of cGVHD clinical symptoms contrib- utes to restrictions on daily activities. For instance, cGVHD can impact the musculoskeletal system, resulting in muscle weakening, discom- fort, and joint stiffness, which limits total functional capacity. Fibro- sis, in particular, might be a major contributor to physical impairment. A patient ability to do normal activities can be harmed by fibrotic changes in the joints and fascia, lung, liver, and skin . It also affects the gastrointestinal tract as well as other organ systems, including oral, esophageal, facial, ophthalmic, neuromuscular system, genitourinary tract and lympho-hematopoietic systems, hair, nail, and genital tissues . Initially, when patients began to have symptoms, they were given no therapy other than supportive care. However, in 1981, Keith Sullivan reported that corticosteroid-based therapy increased survival while controlling symptoms of cGVHD . Over the next three decades, several randomized studies were conducted to enhance initial therapy for cGVHD. Finally, nothing is superior to single-agent prednisone for the treatment of cGVHD, and ruxolitinib has been shown to have superior activity in 2nd line treat- ment of cGVHD compared to other agents. Nevertheless, approxi- mately half of the patients still require 3rd line treatment . In this review article, we summarized the key milestones in the development of BLM chemistry, chemical (synthesis) research and development (CRD), mechanism of action, pharmacokinetics (PK) and pharmacodynamics (PD), nonclinical toxicology, adverse events, regulatory status, and ongoing CT of BLM.
Rho Associated Protein Kinase (ROCK)
Rho-associated protein kinases is a kinase that belongs to the fam- ily of serine-threonine kinases . It is a small GTP-Binding protein and operate via their downstream mediators, Rho-associated protein kinases (ROCK 1 and ROCK2) and regulate various aspects of cell shape, motility, proliferation, and cell death . The structure of ROCKs is made up of an amino-terminal kinase domain, a mid- coiled-coil-forming region with a rho-binding domain (RBD), and a carboxy-terminal cysteine-rich domain (CRD) inside the pleckstrin homology domain (PH) . ROCK inhibitors are recently developed medicines that block the ROCK pathway and thus influence cellular activities. It is beneficial in a variety of diseases, including coronary heart disease, respiratory disease, ocular disease, gastrointestinal disorders, depression, cere- bral cavernous malformation, Alzheimer’s disease, idiopathic pulmo- nary fibrosis, intestinal fibrosis, cancer, erectile dysfunction, insulin resistance, and kidney failure where improper regulation of ROCK activity is essential for disease pathology . Various ROCK inhibitors have been developed so far, including fasudil (not approved by the USFDA but widely used in Japan and China for cerebral vasospasm and cerebral ischemic symptoms fol- lowing subarachnoid haemorrhage surgery), ripasudil (approved by the Japanese PMDA on 26th September 2014, for the treatment of glaucoma and ocular hypertension), netarsudil (approved by the USFDA in December 2017).
ROCK-2 Inhibitors
ROCK-2 is also known as ROK-a and sometimes confusingly called Rho-kinase, located on chromosome 12 and contains 1388 amino acids, ROCK-1 and ROCK-2 share an overall 92% homology in their kinase domains and 65% homology in amino acid sequence . It is used in the regulation of proinflammatory cytokines such as IL-17 and IL-21 and the development of autoimmunity in mice. However, the role of a ROCK-2 signaling pathway in humans is still a mystery. KD025 was the first specific ROCK-2 inhibitor to be described in 2006 and appears to be 100 times more selective for ROCK-2 than ROCK-1 and considerably safer than dual ROCK inhibitors .
Oral treatment of KD025 to healthy human participants reduces T-cell capacity to produce IL-21 and IL-17 by 90% and 60%, respec- tively, but not IFN, in response to T-cell receptor activation in vitro, according to data from a phase 1 clinical study. In T cells derived from healthy subjects or rheumatoid arthritis patients, pharmacolog- ical inhibition with KD025 or siRNA-mediated inhibition of ROCK-2 but not ROCK-1 reduced STAT3 phosphorylation and binding to IL-17 and IL-21 promoters, as well as IFN regulatory factor-4 and nuclear hormone RAR-related orphan receptor T protein levels. In addition, KD025 therapy enhances the suppressive activity of regulatory T cells by increasing STAT5 phosphorylation and positively regulating fork- head box p3 expression. The in vivo injection of KD025 slows the development of collagen-induced arthritis in mice by blocking the Th17-mediated pathway. As a result, ROCK-2 signaling appears to play a role in balancing the proinflammatory and regulatory T-cell subsets. As a result, targeting ROCK-2 in humans may help to restore immunological homeostasis and hence play a role in the therapy of autoimmunity. ROCK-2 inhibitor mechanism has been repre- sented in Fig. 1. A list of ongoing and existing ROCK-1 and ROCK-2 inhibitors is depicted in Table 1.
Milestones in the development of BLM
FDA has approved BLM on 16th July 2021 for the treatment of cGVHD in adults and pediatric patients aged ≥ 12 years after the fail- ure of at least two prior lines of systemic therapy . BLM was developed by Kadmon and Holdings for the marketing of BLM in the United States and the company entered into a partnership with Onco360®, an independent oncology pharmacy.
Chemistry of BLM
BLM is a kinase inhibitor, chemically it is 2-{3-[4-(1H-indazol-5- ylamino)-2-quinazolinyl]phenoxy}-N-(propan-2-yl) acetamide. BLM is available in mesylate form. The molecular formula of BLM mesylate (Fig. 4) is C27H28N6O5S, and the molar mass is 548.62 g/mol. It is a yel- low colour powder that is soluble in dimethylsulfoxide (DMSO), par- tially soluble in methanol and dimethylformamide (DMF), and practically insoluble in water .
Mechanism of action
BLM is a Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor having IC50 values of around 100 nM and 3M for ROCK2 and ROCK1, respectively. In ex vivo and invitro human T cell tests, BLM suppressed pro-inflammatory responses by regulating STAT3/STAT5 phosphorylation and altering the Th17/Treg balance. In vitro, BLM also reduced abnormal pro-fibrotic signaling. In animal models of chronic cGVHD, BLM showed action in vivo.
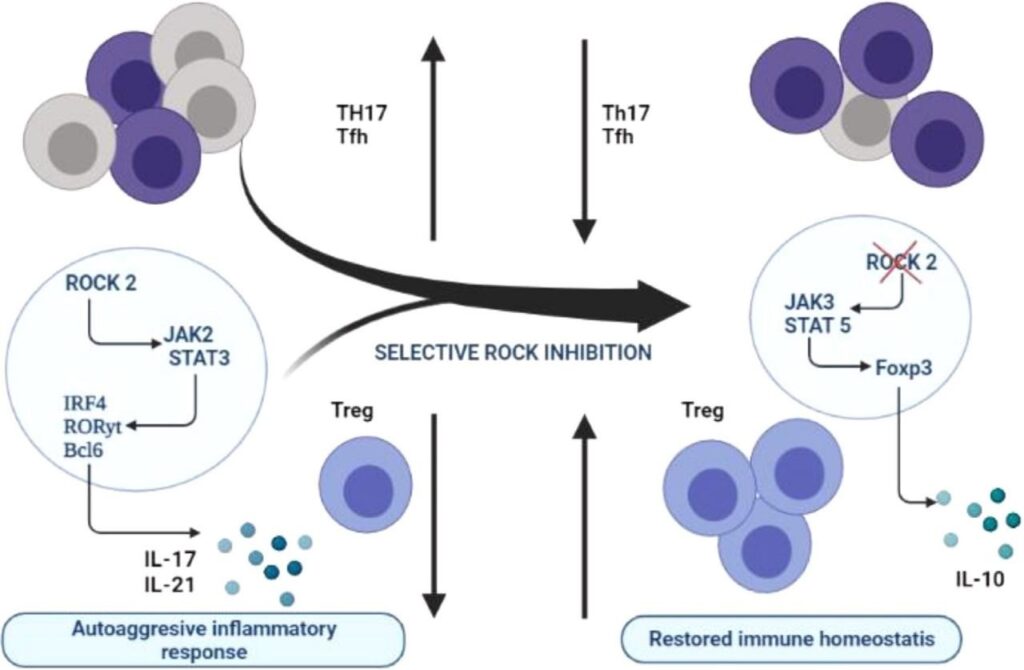
Fig. 1. Mechanism and target of ROCK-2 inhibitor.
Pharmacokinetics (PK)
BLM 200 mg once daily dose produced geometric mean plasma Cmax2390 (44%) ng/ml and the mean (%CV) steady-state AUC 22700 (48%) ng.h/ml. BLM, Cmax, and AUC increased in an approximately proportional manner over a dosage range of 200 to 400 mg (1 to 2 times once daily recommended dosage). The accumulation ratio of BLM was 1.4. Age (18 to 77 years), gender, body weight (38.6 to 143 kg), or mild to moderate renal impairment (eGFR 60 and 90 mL/min/ 1.72m2 to eGFR 30 and 60 mL/min/1.72m2) had no clinically relevant variations in BLM PK. The PK of BLM has not been studied in the pres- ence of severe renal impairment .
Pharmacodynamics (PD)
To prevent and repair the damage caused by cGVHD, BLM appears to suppress various pro-fibrotic and pro-inflammatory processes. Because of BLM, MOA, and findings in animal studies, BLM is to induce embryo- fetal toxicity and might damage a developing fetus if a pregnant woman is exposed. Male patients with female partners of reproductive poten- tial should be advised to take effective contraception during BLM treat- ment and for one week following the final dosage .
Non-clinical toxicology
BLM treated male rats were mated with untreated females, or untreated males were paired with BLM treated females in a combined male and female rat fertility study. BLM was given orally to male rats 70 days before mating and during the mating period, and to female rats 14 days before mating and up to gestation day 7. Administration of BLM to male rats at the dose of 275mg/kg/day caused abnormal sperm findings (lead to reduced motility, reduced sperm count, an increased percentage of abnormal sperm) and testis/epididymis organ changes (degeneration and weight reduction). Fertility was decreased in both male and female rats at the dose of 275mg/kg/day. In general, toxicological investigations, adverse effects in male and female reproductive organs also occurred, findings including decreased follicular development in ovaries at the dose of 275mg/kg/ day in rats and degeneration of spermatozoa at a dose of 35mg/kg/ day in dogs. During the recovery stage, the changes were partially or completely reversed. At dosages of 35 mg/kg/day in dogs and 275 mg/kg/day in rats, the exposure (AUC) is 0.5 times and 8-9 times, respectively, the clinical exposure at the prescribed dose of 200 mg once a day.
Clinical Studies
BLM was explored in ROCKstar (KD025-213, NCT03640481, phase-2) randomized open-label, multicenter pivotal trials to evalu- ate the safety and efficacy of subjects with cGVHD after at least two prior lines of systemic therapy. A total of 166 people with active cGVHD were randomly assigned in a ratio of 1:1 to one of two BLM (KD025) treatment regimens, group A receives BLM 200 mg once a day whereas group B receives 200 mg of BLM twice a day [2,82]. Par- ticipants were excluded from the study if a patient has not been on a consistent regimen of systemic cGVHD therapy for at least two weeks before the screening, in case of histological recurrence of the underly- ing tumor or post-transplant lymphoproliferative disorder, if the patient is treated with ibrutinib (prior ibrutinib therapy is permitted with a 28-day washout period before randomization) at the time of screening.
Subjects are randomized according to prior cGVHD treatment with ibrutinib and the severity of cGVHD. Study participation was terminated in patients who have not developed a response after 12 cycles of BLM treatment, according to the investigator’s judgment if there is no evidence of clinical effectiveness. The effectiveness of BLM was measured by the overall response rate (ORR) through Cycle 7 Day 1, where the overall response includes complete response (CR) or partial response (PR) as per the NIH consensus development proj- ect on CTs in cGVHD guidelines from 2014 .
The overall response rate (ORR) was 75% (95% CI: 63, 85); out of which 6% of patients achieved a complete response whereas 69% achieved a partial response. The average time required to get the first response was 1.8 months (95 % CI: 1.0, 1.9). The median duration of response for chronic cGVHD was 1.9 months (95 % CI: 1.2, 2.9), mea- sured from initial response through progression, death, or new sys- temic treatments. No mortality or new systemic treatment initiation occurred in 62 % (95 % CI: 46, 74) of patients who achieved response for at least 12 months after treatment . The CT data is mentioned in Table 2 . Acronyms: BLM – Belumosudil; dcSSc – Diffuse Cutaneous Systemic Sclerosis; GVHD – Grave Versus Host Disease; cGVHD – Chronic Grave Versus Host Disease; IPF – Idiopathic Pul- monary fibrosis; BA − Bioavailability
Use of BLM in a specific population
Ø Pregnancy
BLM may damage a fetus when given to a pregnant woman, according to data from animal research and the mechanism of action. There are no human data on the administration of BLM in pregnant women to assess for drug-related risk. Inform females and pregnant women of the reproductive potential of the potential risk of BLM treatment to the fetus. The estimated background risk of severe birth abnormalities and miscarriage in clinically recognized pregnancies in the general population of the United States is 2 to 4% and 15 to 20%, respectively.
Animal studies of embryofetal development were carried out in rats using oral doses of BLM of 25, 50, 150, and 300 mg/kg/day in a pilot study and dosages of 15, 50, and 150 mg/kg/day in a pivotal study. Maternal toxicity and embryo-fetal developmental impacts were identified in the pilot study. At 150 and 300 mg/kg/day, mater- nal toxicity (lower body weight growth) was observed. At 50 and 300 mg/kg/day, post-implantation loss was greater. At 50 mg/kg/day, fetal abnormalities such as lack of anus and tail omphalocele, and a dome-shaped head were seen. Rats’ exposure (AUC) at 50 mg/kg/day is almost 3 times that of humans at the recommended dose of 200 mg.
In rabbit embryo-fetal developmental research, pregnant animals were given oral dosages of BLM of 50, 125, and 225 mg/kg/day during the organogenesis phase, which resulted in maternal toxicity and embryo-fetal developmental effects. At dosages less than 125 mg/kg/ day, maternal toxicity (weight loss and death) was reported. At dos- ages of less than 50 mg/kg/day, embryo-fetal effects such as sponta- neous miscarriage, higher post-implantation loss, decreased percentage of live fetuses, abnormalities, and lower fetal body weight was reported. Short tails, ribs that were branching, fused, or mal- formed, sternebrae that were fused, and neural arches that were fused, misaligned, and deformed were among the malformations .
Ø Lactation
There is no information on the presence of BLM or its metabolites in human milk, as well as the effects on a breastfed child or milk pro- duction. Lactating women should not breastfeed throughout BLM treatment and for at least one week after the last dosage due to the risk of significant adverse reactions from BLM in the breastfed child .
Ø Females and males of reproductive potential
When given to a pregnant woman, BLM can damage the fetus. Pregnancy testing can verify the pregnancy status of females of reproductive potential before starting BLM medication. Females of reproductive potential should be advised to use effective contracep- tion while taking BLM and for at least one week following the final dosage. If this medicine is used during pregnancy or if the patient gets pregnant while taking it, the patient should be warned of the risk to the fetus . Males with reproductive-age female partners should use effective contraception during BLM therapy and for at least one week following the final dosage of BLM. Female fertility may be impaired by BLM, according to research on rats. The fertility impact is reversible. It has been shown to reduce male fertility in rats and dogs.
Ø Geriatric use
Out of the 186 participants with cGVHD who participated in BLM clinical trials, 26% were 65 years and older. In comparison to younger individuals, no clinically significant changes in BLM safety or efficacy were identified . The summary of BLM has been depicted in table 3.
Dosage form and strength
Tablet: 200 mg in strength, pale yellow in colour, film, coated, and oblong, labelled with KDM on one side and 200 mg on the other side.
Storage and handling conditions
Tablet containing 200mg of BLM, which is the equivalent of242.5 mg BLM mesylate, Packaged in a bottle of 30 tablets of 200mg (NDC 79802-200-30). It should be stored at 20°C to 25°C (68°F to 77° F), outing stay acceptable since 15°C to 30°C (59°F to 86°F).
Drug interactions
Strong CYP3A inducers: BLM co-administration with strong CYP3A inducers reduces BLM exposure, which may reduce the effi- cacy of BLM. Increase the dose of BLM when administered along with strong CYPA3 inducers. Strong CYP3A Inhibitors: there is no clinical impact on the PK of BLM when co-administered with Strong CYP3A inhibitors (e.g., Itraconazole). Moderate CYP3A inducers: when mod- erate CYP3A inducers are co-administered along with BLM, it may decline the efficacy of BLM. CYP2C9 Substrate: No clinical impact on BLM when co-administered with CYP2C9 substrate. Proton pump inhibitors: Co-administration of BLM with PPIs (e.g., Rabeprazole) reduces the efficacy of BLM .
Adverse events
Adverse reactions (All grades) occurred in patients with cGVHD treated with BLM in the phase II clinical trials NCT03640481 (KD025- 213) and NCT02841995 (KD025-208) in 83 subjects (N=83) included non-specified pathogen infection (53%), viral infection(19%), bacterial infection (16%), decreased appetite (17%), rashes (12%), pruritis (11%). Grade 3 to 4 adverse events included nonspecified pathogen infection (16%), viral infection (4%), bacterial infection (4%), asthenia (4%), edema (1%), pyrexia(1%), nausea (4%), diarrhea (5%), abdominal pain (1%), dyspnea (5%), hemorrhage (5%), hypertension (7%), musculo- skeletal pain (4%), arthalgia (2%), decreased appetite (1%) . Laboratory abnormalities Grade 0-1 baseline included decreased phosphate (76), increased gamma-glutamyl transferase (47), decreased calcium (82), increased alkaline phosphate (80), increased creatinine(83), increased potassium (82), increased alanine amino- transferase (83%), decreased lymphocytes (62), decreased hemoglo- bin (79), decreased platelet levels (82), decreased neutrophil levels (83). Grade 3-4 maximum post included decreased phosphate (7%), increased gamma-glutamyl transferase (11%), decreased calcium (1%), increased alanine aminotransferase (2%), increased potassium (1%), decreased lymphocytes (13%), decreased hemoglobin (1%), decreased platelet levels (5%), decreased neutrophil levels (4%) . A serious adverse reaction was reported in one patient with severe nausea, vomiting, diarrhoea, and multiorgan failure. (Table 4) .
Regulatory status
BLM received the FDA approval on 16th July 2021 for the treat- ment of cGVHD in adult and pediatric patients after failure of at least two prior lines of systemic therapy and on 9th August 2020 FDA granted orphan drug status to BLM for the treatment of systemic scle- rosis . On 17th October 2019, the EU also granted orphan drug status to BLM for the treatment of cGVHD .
Ongoing clinical trials
Phase-II study (NCT04680975, KD025-215), evaluating the activ- ity of BLM for the management of diffuse cutaneous systemic sclero- sis (dcSSc), is ongoing and initial data is expected by the end of 2021 . Phase-II (NCT03919799, KD025-209) randomized, placebo-con- trolled study of BLM for the treatment of dcSSc is continuing . Phase-I (NCT04166942, KD025-109) single-dose open-label, non- randomized, parallel-group study for BLM is ongoing for the treat- ment of hepatic impairment. The FDA agrees that no dose adjust- ments are required by age, body size or renal function. However, the FDA does not agree that no dose adjustments are necessary for con- comitant CYP3A inducers and concomitant PPIs. For hepatic impairment, there is insufficient information to determine the need for dose adjustments .
Conclusion
BLM is the first and only approved drug that targets ROCK 2 signal- ing pathway that regulates inflammatory response and fibrotic pro- cesses. It is approved by the USFDA for the treatment of cGVHD in adults and pediatric patients aged ≥ 12 years after the failure of at least two prior lines of systemic therapy. The USFDA approval is based on the evidence of the ROCKstar study (KD025-213), which was a randomized, open-label, multicenter pivotal trial of BLM in patients with cGVHD who had undergone two to five lines of systemic treatment. BLM 200 mg dose was given to 65 participants daily. The median period from diagnosis to treatment for cGVHD was 25.3 months, and 48% of patients had four or more organs affected. Patients had received a median of three prior lines of systemic therapy, with 78% of them resis- tant to their most recent treatment. Through cycle 7 and day 1 of ther- apy, BLM 200 mg once a day had a 75 % ORR (95 % CI: 63, 85), with 6% having a complete response and 69% achieving a partial response. The average time it took to get a first response was 1.8 months. 62% of par- ticipants did not need new systemic therapy for at least a year after their response. The median duration of response for cGVHD was 1.9 months, measured from the initial response through progression, death, or new systemic treatments. The lee symptom scale (LSS) score, a cGVHD symptom assessment, improved clinically significantly from baseline in 52% of patients through cycle 7 day 1 of therapy, showing ORR. BLM was well tolerated, with side effects similar to those seen in patients with severe cGVHD who are taking corticosteroids and/or other immu- nosuppressive drugs. BLM patients reported significant improvements in cGVHD symptoms, demonstrating that the therapy not only improved organ responses but also helped them to reduce symptom burden. For a chronic illness with a significant symptom load, this is critical.
Expert opinion
FDA approval and assimilation of BLM in the treatment of cGVHD denotes substantial advancement in this disorder. Compared in con- tradiction of chronological outcomes, the 75% ORR represents an enhancement. For cGVHD, while BLM is a satisfactory option, investi- gation for new therapeutic agents with higher relative response con- tinues a research priority. Possibly the greatest advantages of BLM treatment are its ease of administration and comparatively adaptable toxicity. BLM Oral administration signifies a substantial development all over several recent options. In summary, BLM likely signifies a novel standard in cGVHD and is an important therapeutic agent for transplant physicians and clinicians to reduce the possibly extremely substantial complication post-transplantation.
